Overview
Physical models representing molecular architectures of chemical compounds play essential roles in understanding chemistry. The use of molecular models makes it easier to visualize the structures and shapes of atoms and molecules.
Skeletal Model
Simpler two-dimensional representations of chemical compounds are accomplished using skeletal models. The illustration shows only the molecular framework or bonds without explicitly showing the atoms. In this representation, many of the carbon atoms and hydrogen atoms are not explicitly shown. However, the positions of atoms are implied by the junctions or ends of the bonds. This model helps to represent larger and more complex chemical structures.
Ball-and-stick Model
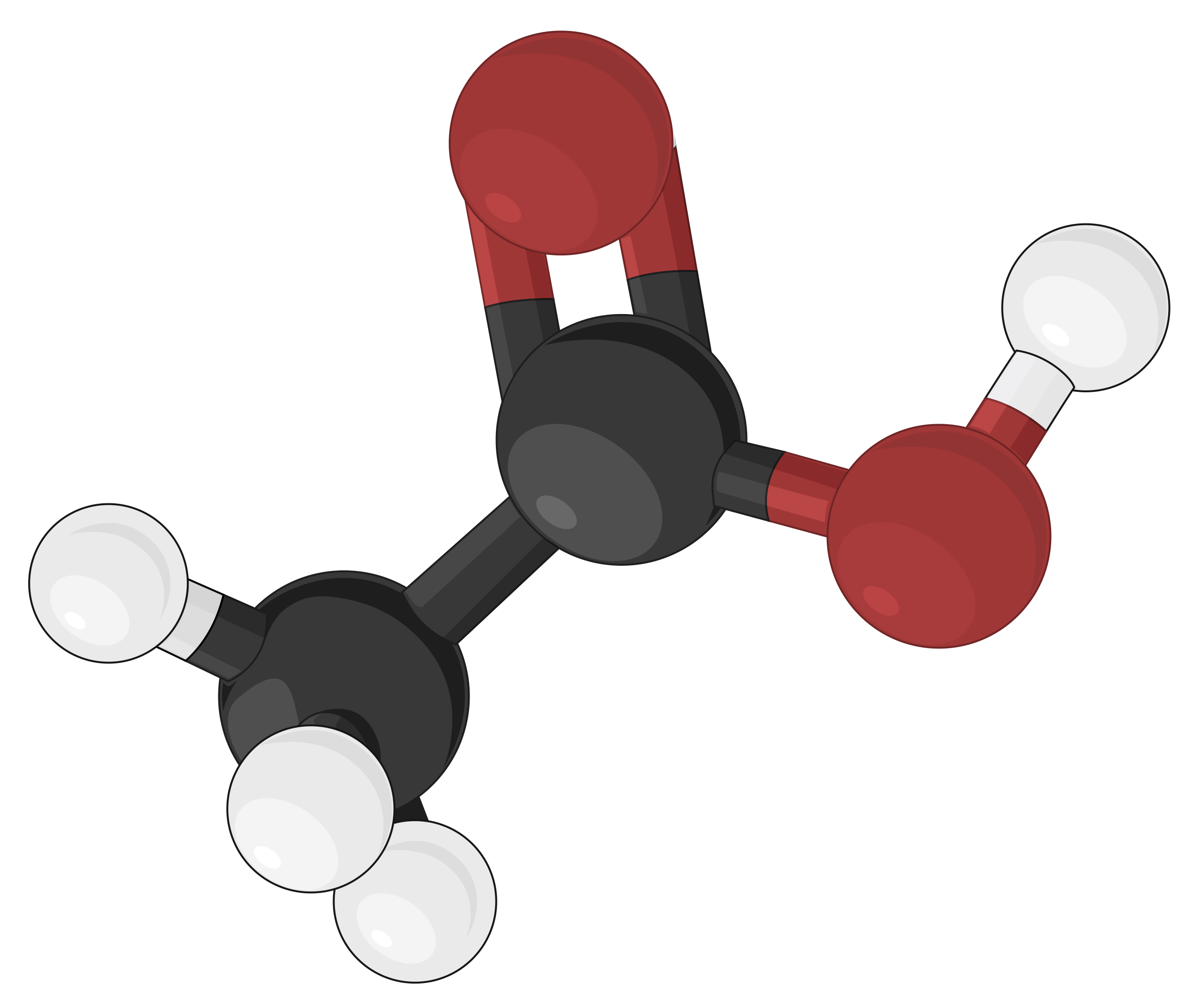
Ball-and-stick models are three-dimensional models, where the atoms are depicted as color-coded balls or spheres, specific to different elements. The chemical bonds that connect the atoms are represented by rods and are easier to visualize. In doing so, the sizes of the balls are made relatively smaller, thereby compromising on the proportional correlation with the actual atomic size. Yet, the ball-and-stick model defines the angles between atoms, clearly depicting the molecular geometry of simple to more complex structures as compared to other molecular models.
Space-filling Model
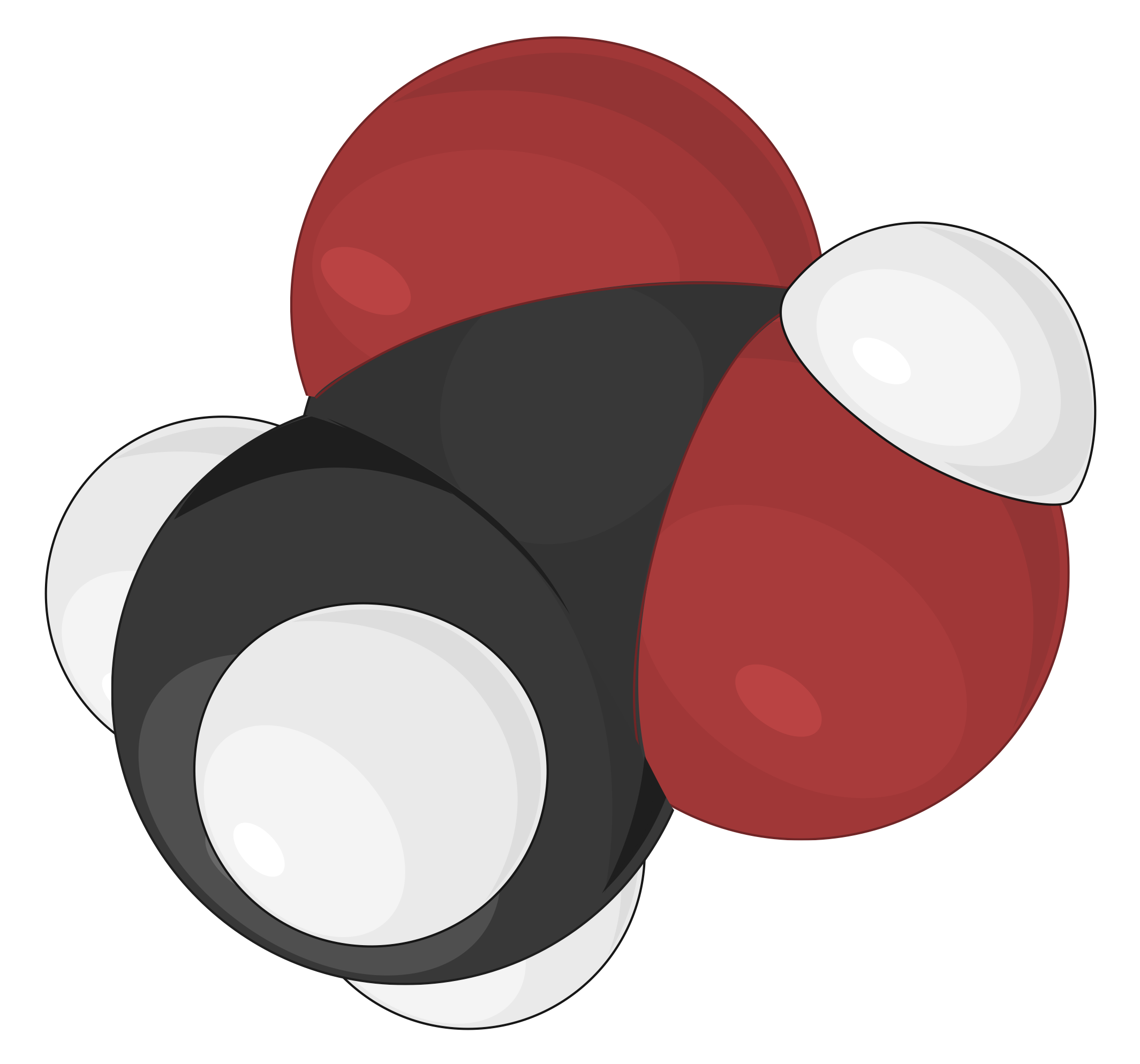
Space-filling models are most realistic, where the atoms are scaled up in size to fill the space between each other. The size and position of an atom in this model are determined by its bonding properties and van der Waals radius, or contact distance. The van der Waals radius describes how closely two atoms can approach each other when a covalent bond does not link them. The spheres in this model illustrate the relative space occupied by each atom within a compound, while the angles between atoms are not clearly visible
First designed by chemists Robert Corey and Linus Pauling, and later improved by Walter Koltun, the CPK coloring convention designates specific colors to atoms of each element. For example, according to the CPK convention, all hydrogen atoms are colored white, carbon atoms are black, nitrogen atoms are blue, oxygen atoms are red, sulfur atoms are deep yellow, and phosphorus atoms are purple. Alkaline earth metals are represented by dark green, and alkali metals are indicated by violet.
As an example, different molecular models of acetic acid (CH3COOH) can be represented in the following ways:
 |
 |
|
| Skeletal model | Ball-and-stick model | Space-filling model |
This text is adpted from: Openstax, Chemistry 2e, Section 2.4: Chemical Formulas.
Procedure
The composition and molecular architecture of a chemical substance can be effectively visualized and better understood by using three-dimensional molecular models.
The ball-and-stick model and space-filling model are two standard types of molecular models that show the geometric arrangement of atoms in a chemical compound. These models are built as either physical objects, made of plastic or wood, or virtual computer simulations.
The ball-and-stick model utilizes spheres or balls to depict atoms. Sticks or rods connecting the spheres represent the chemical bonds, and the angle between the rods matches the bond angle within the actual compound. Two or three rods typically represent double and triple bonds, respectively.
The distance between the centers of each ball is proportional to the exact distance between the corresponding atomic nuclei.
The balls are typically color-coded to distinguish atoms of different elements. The colors are assigned to comply with the CPK coloring convention introduced by the chemists Robert Corey, Linus Pauling, and Walter Koltun. For example, white, black, and red balls represent hydrogen, carbon, and oxygen atoms, respectively.
The space-filling model is more realistic and utilizes full-sized spheres to represent the atoms. The spheres, however, mask the chemical bonds and the bond angles present between the atoms.
The spheres are made proportionate to the relative sizes of atoms and provide a clear insight about the actual appearance and space occupied by each atom if scaled to visible size.
The color-codes used in this space-filling model also follow the CPK coloring convention.