Overview
While the differential rate law relates the rate and concentrations of reactants, a second form of rate law called the integrated rate law relates concentrations of reactants and time. Integrated rate laws can be used to determine the amount of reactant or product present after a period of time or to estimate the time required for a reaction to proceed to a certain extent. For example, an integrated rate law helps determine the length of time a radioactive material must be stored for its radioactivity to decay to a safe level.
Using calculus, the differential rate law for a chemical reaction can be integrated with respect to time to give an equation relating the reactant/product amount to the elapsed time of the reaction.
First-Order Reactions
Integration of the rate law for a simple first-order reaction (rate = k[A]) results in an equation describing the variation in reactant concentration with time:
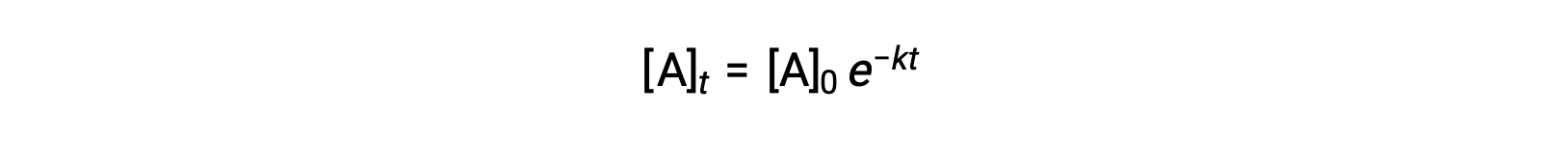
Here, [A]t is the concentration of A at any time t, [A]0 is the initial concentration of A, and k is the first-order rate constant. For mathematical convenience, this equation is rearranged to a format showing a linear dependence of concentration on time that takes the form of a straight-line equation (y = mx + b):
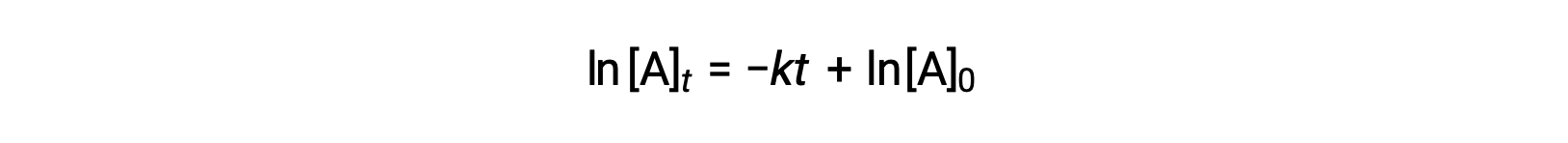
The equation suggests that a plot of ln[A]t versus t for a first-order reaction is a straight line with a slope of −k and a y-intercept of ln[A]0. If a set of rate data are plotted in this fashion but do not result in a straight line, the reaction is not first order in A.
Second-Order Reactions
The differential rate law for a simple second-order reaction is rate = k[A]2, and the integrated rate law is:
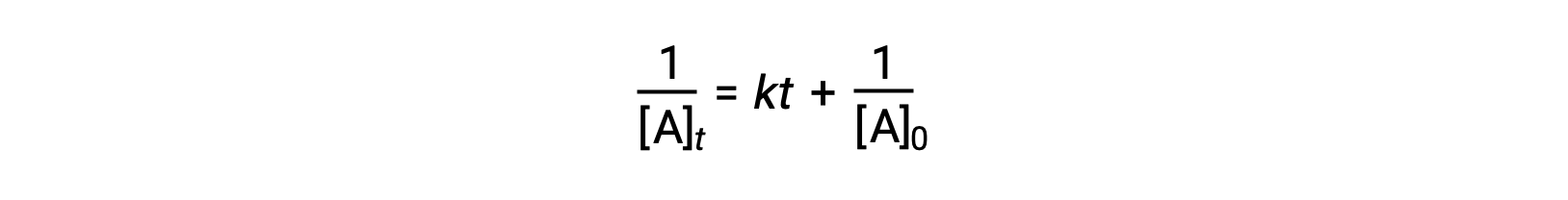
The second-order integrated rate law also takes the form of the equation for a straight line. According to the equation, a plot of 1/[A]t versus t for a second-order reaction is a straight line with a slope of k and a y-intercept of 1/[A]0. If the plot is not a straight line, then the reaction is not second-order.
Zero-Order Reactions
For zero-order reactions, the differential rate law is rate = k. A zero-order reaction exhibits a constant reaction rate, regardless of the concentration of its reactant(s). Zero-order kinetics are observed for some reactions only under certain specific conditions. These same reactions exhibit different kinetic behaviors when the specific conditions aren’t met, and for this reason, the more prudent term pseudo-zero-order is sometimes used.
The integrated rate law for a zero-order reaction is also a linear function, taking the form of y = mx + b:
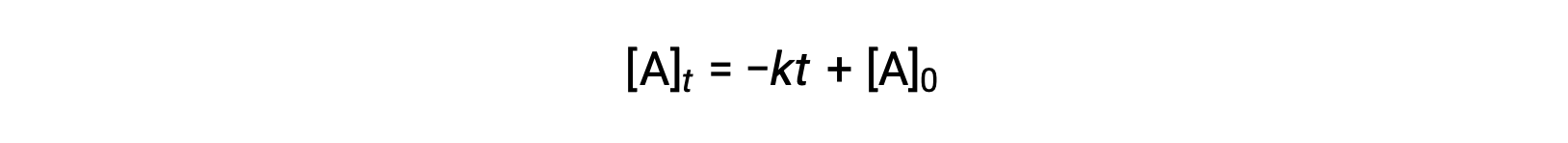
A plot of [A] versus time t for a zero-order reaction is a straight line with a slope of −k and a y-intercept of [A]0.
This text is adapted from Openstax, Chemistry 2e, Section 12.4: Integrated Rate Laws.
Procedure
A rate law used to determine the reaction rate from reactant concentrations and rate constants can be converted into rate laws demonstrating the dependence of reaction rate on reactant concentration and time.
These rate laws can be used to study how slowly or quickly a reactant is consumed, or how much time is necessary to reach half the concentration of a reactant.
To begin, examine the differential rate law, which expresses the reaction rate as a change in reactant concentration during a specific time interval. Integration of this law leads to the integrated rate law, which expresses the reaction rate as a relation between a reactant’s initial concentration and its concentration after a specific duration.
The integrated rate law is dependent on the overall reaction order and, hence, varies for each reaction type. However, irrespective of the overall order, all integrated rate laws take the form of a standard linear equation with distinct y, m, x, and b components, and can be plotted to generate a straight line.
In a zero-order integrated rate law, [A]t is the reactant concentration at the time t, k is the rate constant, t is the time, and [A]0 is the initial reactant concentration.
For a zero-order reaction, a plot of the reactant concentration as a function of time generates a straight line. The slope is the negative value of the rate constant, and the y-intercept is the initial reactant concentration.
In a first-order reaction, the natural log of reactant concentration plotted as a function of time gives a straight line. The slope corresponds to the negative value of the rate constant, while the y-intercept gives the natural log of the initial reactant concentration.
According to the second-order integrated rate law, a plot of the inverse of the reactant concentration versus time yields a straight line. The slope equates to the rate constant, and the y-intercept represents the inverse of the initial reactant concentration.
The overall reaction order can be identified using experimental kinetic data by plotting the different integrated rate laws. Only the plot with a linear graph corresponds to the correct overall reaction order. Subsequent analysis allows determining the rate constant and reactant concentration at any given time.