Overview
Rate laws describe the relationship between the rate of a chemical reaction and the concentration of its reactants. In a rate law, the rate constant k and the reaction orders are determined experimentally by observing how the rate of reaction changes as the concentrations of the reactants are changed. A common experimental approach to the determination of rate laws is the method of initial rates. This method involves measuring reaction rates for multiple experimental trials carried out using different initial reactant concentrations. Comparing the measured rates for these trials permits determination of the reaction orders and, subsequently, the rate constant, which together are used to formulate a rate law.
The rate of a reaction, for example, involving nitric oxide with ozone [NO (g) + O3 (g) ⟶ NO2 (g) + O2 (g)] can be determined from the experimental data of method of initial rates, in the laboratory.
| Trial | [NO] (mol/L) | [O3] (mol/L) | Δ[NO2]/Δt (mol/L·s) |
| 1 | 1.00 × 10−6 | 3.00 × 10−6 | 6.60 × 10−5 |
| 2 | 1.00 × 10−6 | 6.00 × 10−6 | 1.32 × 10−4 |
| 3 | 1.00 × 10−6 | 9.00 × 10−6 | 1.98 × 10−4 |
| 4 | 2.00 × 10−6 | 9.00 × 10−6 | 3.96 × 10−4 |
| 5 | 3.00 × 10−6 | 9.00 × 10−6 | 5.94 × 10−4 |
From the rate data, a generic rate law; rate = k[NO]m[O3]n is formulated. The values of the reaction orders m and n, and rate constant k are determined from the experimental data using a three-part process:
In step 1, the value of m is determined from the data in which [NO] varies, and [O3] is constant. In trials 3, 4 and 5, [NO] varies while [O3] remains constant. When [NO] doubles from trial 3 to 4, the rate doubles, and when [NO] triples from trial 3 to 5, the rate also triples. Thus, the rate is also directly proportional to [NO], and m in the rate law is equal to 1.
In step 2, the value of n is determined from data in which [O3] varies, and [NO] is constant. In trials 1,2 and 3, [NO] is constant and [O3] varies. The reaction rate changes in direct proportion to the change in [O3]. When [O3] doubles from trial 1 to 2, the rate doubles; when [O3] triples from trial 1 to 3, the rate increases also triples. Thus, the rate is directly proportional to [O3], and n is equal to 1. The rate law is thus: rate = k [NO]1 [O3]1 = k [NO][O3]
In step 3, the value of k is determined from one set of concentrations (for instance, the data from trial 1) and its corresponding rate.
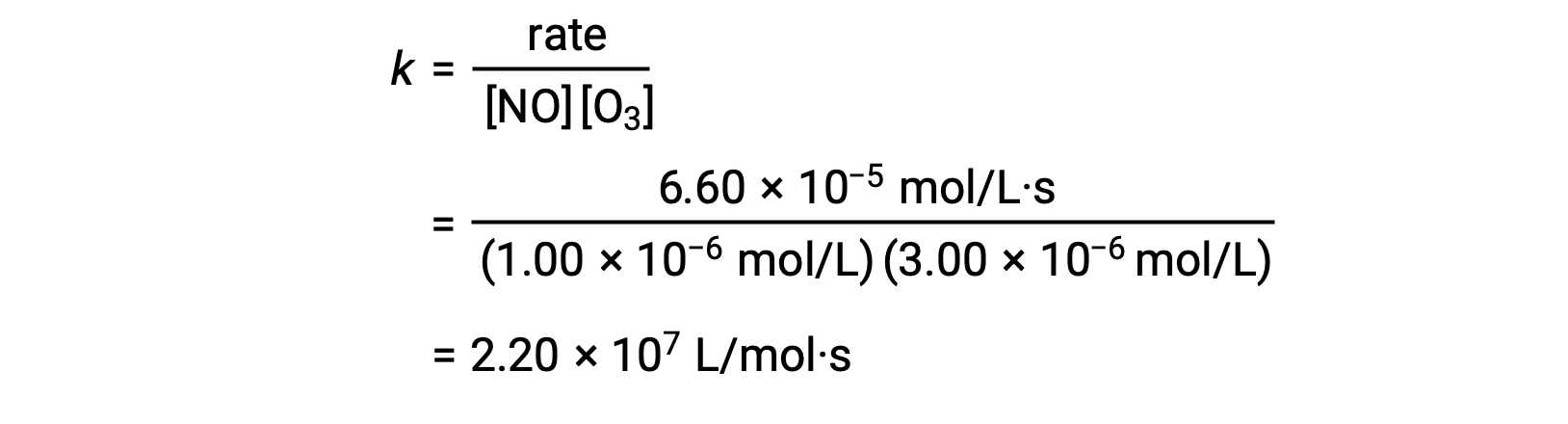
In reactions where the data from the method of initial rates does not directly imply the relation between initial concentrations and initial rates, a calculation involving the ratio of rate laws can be employed to calculate the reaction order and rate constant.
For example, the general rate law for the reaction 2 NO (g) + Cl2 (g) ⟶ 2 NOCl (g) is expressed as: rate = k [NO]m [Cl2]n.
The data from the method of initial rates are:
| Trial | [NO] (mol/L) | [Cl2] (mol/L) | Initial Rate (mol/L·s) |
| 1 | 0.10 | 0.10 | 0.00300 |
| 2 | 0.10 | 0.15 | 0.00450 |
| 3 | 0.15 | 0.10 | 0.00675 |
The values of m and n can be determined from the experimental data using an algebraic approach, following which the value of k is determined.
In step 1, the value of m is determined from the data in which [NO] varies and [Cl2] is constant. A ratio of rate laws is expressed by substituting data from two different trials (for instance trial 3 and trial 1).
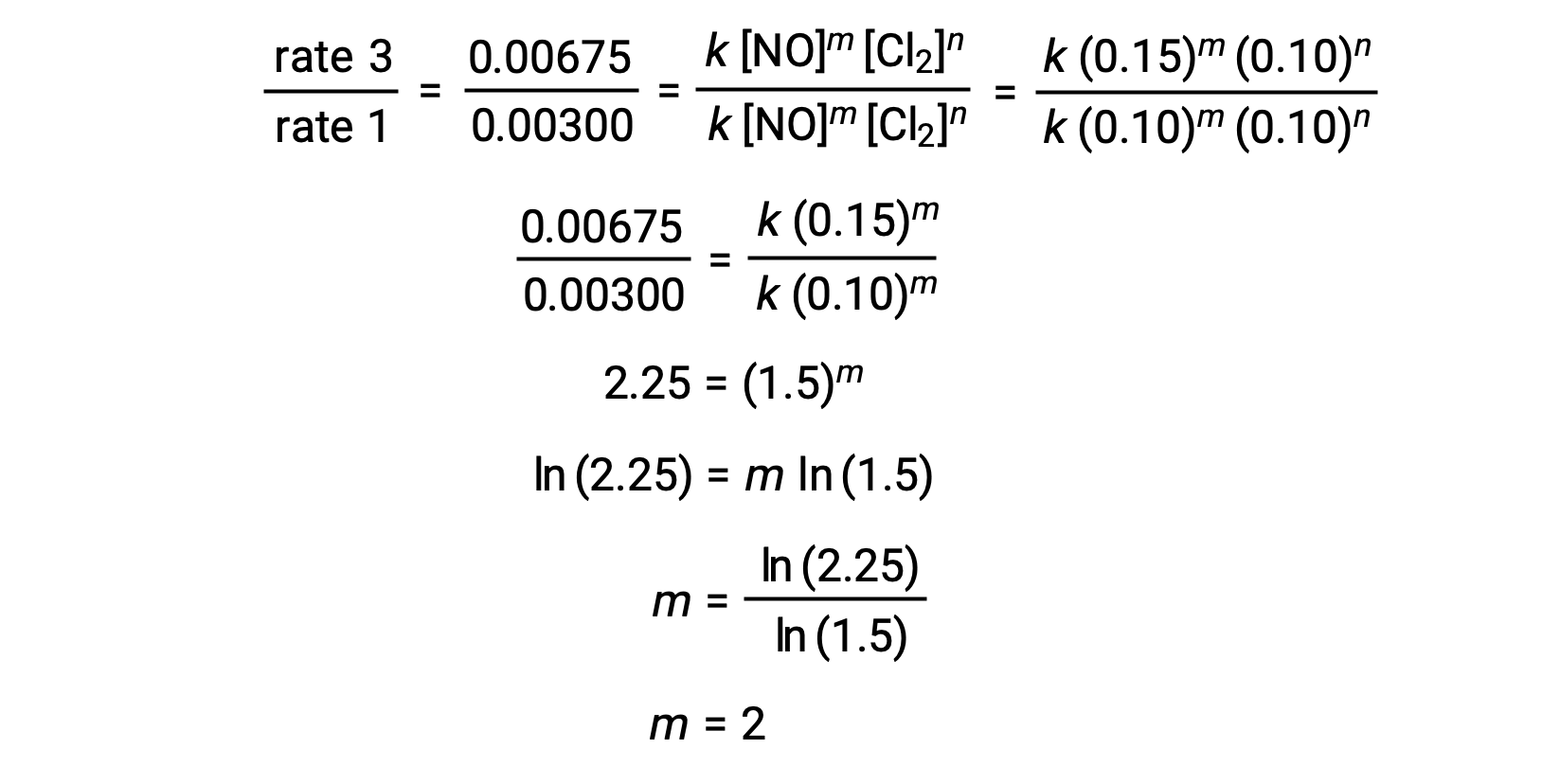
In step 2, the value of n is determined from data in which [Cl2] varies, and [NO] is constant.
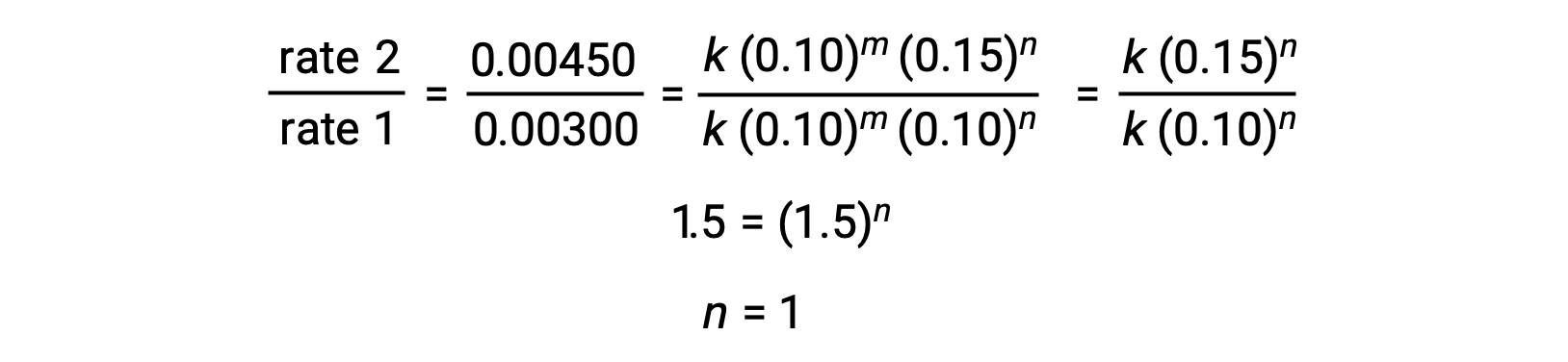
Using the computed values of m and n the rate law is expressed as rate = k [NO]2 [Cl2].
In step 3, the numerical value of the rate constant k is determined with appropriate units. The units for the rate of a reaction are mol/L·s. The units for k is concluded by substituting the units of all other parameters in the rate law. In this example, the concentration units are mol3/L3. The units for k should be L2/mol2·s so that the rate is in terms of mol/L·s. The value of k is determined once the rate law expression has been solved, by simply substituting the values from any of the experimental trials (for instance trial 1).
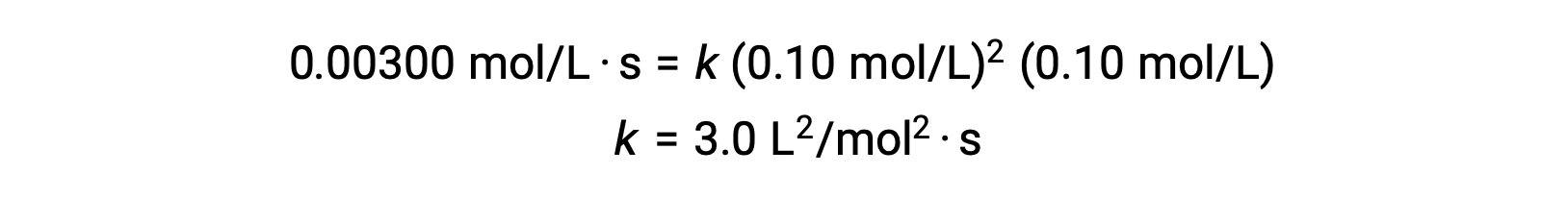
Reaction Order and Rate Constant Units
In some reactions, the reaction orders in the rate law happen to be the same as the coefficients in the chemical equation for the reaction. This is merely a coincidence and very often not the case. Rate laws may exhibit fractional orders for some reactants, and negative reaction orders are sometimes observed when an increase in the concentration of one reactant causes a decrease in reaction rate. Rate laws are determined by experiment only and are not reliably predicted by reaction stoichiometry.
The units for a rate constant will vary as appropriate to accommodate the overall order of the reaction. The unit of the rate constant for a zero-order reaction is mol/L·s (or M/s) and that for a first-order reaction is 1/s. The unit of the rate constant for a second-order reaction is L/mol·s (or 1/M·s) and that for a third-order reaction is L2/mol2·s. Although the specific units for concentration and time are indicated as (mol/L) and (s), any other valid units can be used to represent the properties of concentration and time.
This text is adapted from Openstax, Chemistry 2e, Section 12.3: Rate Laws.
Procedure
For a chemical reaction, the rate law expresses the relationship between the reaction rate and reactant concentration. The exponents of the reactant concentrations influence the reaction rate individually and are called reaction orders.
The reaction order is determined experimentally by employing the method of initial rates, where a chemical reaction is repeated multiple times with varying reactant concentrations to measure the initial reaction rates.
An increase in the reactant concentration, producing a linearly-proportional increase in the reaction rate, characterizes a first-order reaction. If doubling the reactant concentration quadruples the initial rate, then a second-order reaction is observed.
However, if the change of reactant concentrations does not affect the initial rate values, then a zero-order reaction is observed.
When the obtained data of initial rates present an indiscernible relationship between the change in initial reactant concentration and the corresponding rate, a ratio of rate laws is calculated.
Here, any two concentration values of the reactant and their corresponding reaction rates are used to determine the reaction order.
But what about reactions with multiple reactants? First, using the method of initial rates, the reaction order of each reactant is determined individually.
Next, the individual reaction orders are expressed as exponents to their respective reactant concentrations to formulate the rate law. Lastly, the summation of individual exponents from the rate law determines the overall reaction order.
While the reaction order depicts the reaction rate’s dependence on the reactant concentration, a direct measure of the relative reaction speed is indicated by the rate constant.
The rate constant k, is the proportionality coefficient relating the reaction rate to the product of reactant concentrations.
The unit of a rate constant depends on the overall reaction order and can be determined by rearranging the rate law to solve for the rate constant. For a zero-order reaction, k has the unit molarity per seconds. The unit for a first-order reaction is 1/s, and for a second-order reaction it is 1/M·s.
A large rate constant indicates a fast reaction. Conversely, a smaller rate constant indicates a slow reaction. A value of zero signifies the absence of any chemical reaction.