Overview
Chemical Kinetics
The reaction rate is the speed at which a chemical reaction occurs. The reaction rate is defined as the change in concentration of a component in the reaction with time. The speed of a reaction depends on several factors, including the concentration of reactants and the temperature at which the reaction is performed. Each reactant contributes to the speed of the reaction by a specific factor. This relationship is defined by the reaction rate law.
Rate Law
The rate law is an equation that describes the relationship between the concentration of reactants, A and B, and their reaction orders, m and n. The rate constant, k, relates the concentrations and orders of the reactants to the reaction rate. It is dependent on the reaction as the temperature at which the reaction is performed.
r = k [A]m[B]n for aA + bB → cC
Arrhenius Equation
The Arrhenius equation relates the reaction rate constant to the activation energy of a chemical reaction. The activation energy is defined as the amount of energy a chemical reaction needs in order to proceed. If a reaction does not meet this activation energy requirement, the reaction will not proceed.
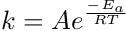
The negative exponential relationship between k and the temperature indicates that as temperature increases, the value of k also increases. Since the rate constant can be determined experimentally over a range of temperatures, the activation energy can be calculated using the Arrhenius equation. By taking the natural logarithm of both sides, the Arrhenius equation is rewritten as a linear equation.
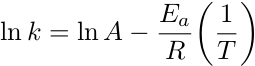
A plot of ln k vs. 1/T yields a straight line with a slope equal to -Ea/R and a y-intercept of ln A. Since the ideal gas constant, R, is known, Ea can be determined graphically using a series of k values at different temperatures.
Catalysts and Activation Energy
Some chemical reactions have a sufficiently large activation energy that makes the reaction proceed slowly, if at all. The decomposition reaction of hydrogen peroxide into oxygen and water occurs spontaneously, but it occurs at an incredibly slow rate. One way to overcome this initial barrier is to supply energy in the form of heat. However, this is not always ideal as excessive heat may affect the stability of the products or reactants or may facilitate side reactions.
The activation energy for chemical reactions can be altered using catalysts. A catalyst lowers the activation energy of a chemical reaction, but it is not consumed by the reaction. In other words, a catalyst facilitates the chemical reaction by making it easier to overcome the critical activation energy requirement. In the decomposition of hydrogen peroxide, the addition of iron nitrate lowers the activation energy and allows the reaction to proceed at a faster rate. However, it is important to note that while a catalyst may affect the rate of a reaction, a catalyst DOES NOT change the amount of product produced by the reaction.
References
- Kotz, J.C., Treichel Jr, P.M., Townsend, J.R. (2012). Chemistry and Chemical Reactivity. Belmont, CA: Brooks/Cole, Cengage Learning.
- Silberberg, M.S. (2009). Chemistry: The Molecular Nature of Matter and Change. Boston, MA: McGraw Hill.
Procedure
The measure of how fast a reaction proceeds is called the reaction rate. The rate of a chemical reaction is defined by the rate law, which describes the relationship between the speed of the reaction and the reactant concentrations. In this equation, k is the rate constant, A and B are the two reactants, and m and n are their respective reaction orders.
The rate constant converts the relationship to the proper units of rate, moles per liter per second. Thus, the rate constant has different units, depending on the overall order of the reaction. However, the rate constant holds more significance than simply unit conversion. The rate constant is related to the minimum amount of energy required for a chemical reaction to occur - called the activation energy.
In a reaction, the reactants are at an initial state of potential energy. As the reaction proceeds, it must overcome a certain potential energy, the activation energy, before reaching its final state. The net energy of the reaction is the difference between the initial and final states. This difference can be negative, meaning that the reaction releases energy, or positive, meaning that it absorbs energy.
If there is not enough energy available to overcome the activation energy, the reaction will not proceed. In some cases, energy can be supplied in the form of heat. This provides additional energy to overcome the barrier to activation, and the reaction can proceed. A catalyst may also be added, which provides an alternative lower activation energy pathway between the reactants and products.
Catalysts are not consumed in the reaction and, therefore, do not affect the net energy of the reaction. The activation energy is determined experimentally, and it is related to the reaction constant k by the Arrhenius equation where A is the pre-exponential or frequency factor, R is the universal gas constant, and T is the absolute temperature at which the reaction occurs.
From this equation, we know that increasing the reaction temperature or decreasing the activation energy increases the rate constant. Going back to the rate law equation, it follows that a higher rate constant results in a higher reaction rate. This makes sense because as temperature increases, molecules move faster and collide more frequently, resulting in an increased fraction of molecules with higher energy than the activation energy.
In this lab, you will learn how to measure the activation energy of a reaction experimentally using the decomposition of hydrogen peroxide as the model reaction.