Overview
The spontaneity of a process depends upon the temperature of the system. Phase transitions, for example, will proceed spontaneously in one direction or the other depending upon the temperature of the substance in question. Likewise, some chemical reactions can also exhibit temperature-dependent spontaneities. To illustrate this concept, the equation relating free energy change to the enthalpy and entropy changes for the process is considered:
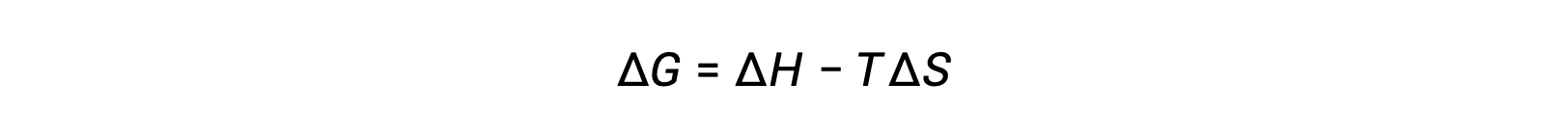
The spontaneity of a process, as reflected in the arithmetic sign of its free energy change, is then determined by the signs of the enthalpy and entropy changes and, in some cases, the absolute temperature. Since T is the absolute (kelvin) temperature, it can only have positive values. Four possibilities, therefore, exist with regard to the signs of the enthalpy and entropy changes:
- Both ΔH and ΔS are positive. This condition describes an endothermic process that involves an increase in system entropy. In this case, ΔG will be negative if the magnitude of the TΔS term is greater than ΔH. If the TΔS term is less than ΔH, the free energy change will be positive. Such a process is spontaneous at high temperatures and nonspontaneous at low temperatures.
- Both ΔH and ΔS are negative. This condition describes an exothermic process that involves a decrease in system entropy. In this case, ΔG will be negative if the magnitude of the TΔS term is less than ΔH. If the TΔS term’s magnitude is greater than ΔH, the free energy change will be positive. Such a process is spontaneous at low temperatures and nonspontaneous at high temperatures.
- ΔH is positive, and ΔS is negative. This condition describes an endothermic process that involves a decrease in system entropy. In this case, ΔG will be positive regardless of the temperature. Such a process is nonspontaneous at all temperatures.
- ΔH is negative, and ΔS is positive. This condition describes an exothermic process that involves an increase in system entropy. In this case, ΔG will be negative regardless of the temperature. Such a process is spontaneous at all temperatures.
This text is adapted from Openstax, Chemistry 2e, Section 16.4: Free Energy.
Procedure
For a reaction to be spontaneous at constant temperature and pressure, the change in Gibbs free energy, ΔG, must be less than zero.
The sign of ΔG depends on the signs and the relative values of enthalpy, entropy, and temperature.
Enthalpy favors spontaneity when the reaction releases heat to the surroundings, while entropy favors spontaneity when there is more disorder in the system.
If ΔH is negative and ΔS is positive, as in the reaction between sodium hydroxide and hydrochloric acid, ΔG is negative at all temperatures. Thus, exothermic reactions—where the entropy of the system increases—are always spontaneous.
If both ΔH and ΔS are negative, ΔG depends on the temperature. Consider the freezing of water into ice, an exothermic reaction where the entropy of the system decreases.
At temperatures below the freezing point of water, the water will freeze spontaneously, releasing heat and becoming more ordered. Thus, reactions with negative enthalpy and entropy changes are spontaneous only at low temperatures.
ΔG is also dependent on temperature if both ΔH and ΔS are positive.
A common example is a chemical cold pack, where solid ammonium nitrate dissolves in water, that absorbs heat from the surroundings. This endothermic reaction proceeds spontaneously at room temperature due to the increase in disorder of the system. Thus, reactions with positive enthalpy and entropy changes are spontaneous only at higher temperatures.
If the temperature were lowered such that the TΔS becomes smaller than ΔH, ΔG would be positive, and the reaction would become nonspontaneous.
When ΔH is positive and ΔS is negative, ΔG is always positive, and the reaction is nonspontaneous at all temperatures.