Procedure
Buffer solutions do not have an unlimited capacity to keep the pH relatively constant . Instead, the ability of a buffer solution to resist changes in pH relies on the presence of appreciable amounts of its conjugate weak acid-base pair. When enough strong acid or base is added to substantially lower the concentration of either member of the buffer pair, the buffering action within the solution is compromised.
The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the pH changes significantly, usually by one unit. Buffer capacity depends on the amounts of the weak acid and its conjugate base that are in a buffer mixture. For example, 1 L of a solution that is 1.0 M in acetic acid and 1.0 M in sodium acetate has a greater buffer capacity than 1 L of a solution that is 0.10 M in acetic acid and 0.10 M in sodium acetate even though both solutions have the same pH. The first solution has more buffer capacity because it contains more acetic acid and acetate ion.
Selection of Suitable Buffer Mixtures
There are two useful rules of thumb for selecting buffer mixtures:
- A good buffer mixture should have about equal concentrations of both of its components. A buffer solution has generally lost its usefulness when one component of the buffer pair is less than about 10% of the other.
- Weak acids and their salts are better as buffers for pHs less than 7; weak bases and their salts are better as buffers for pHs greater than 7.
Blood is an important example of a buffered solution, with the principal acid and ion responsible for the buffering action being carbonic acid, H2CO3, and the bicarbonate ion, HCO3−. When a hydronium ion is introduced to the bloodstream, it is removed primarily by the reaction:

An added hydroxide ion is removed by the reaction:
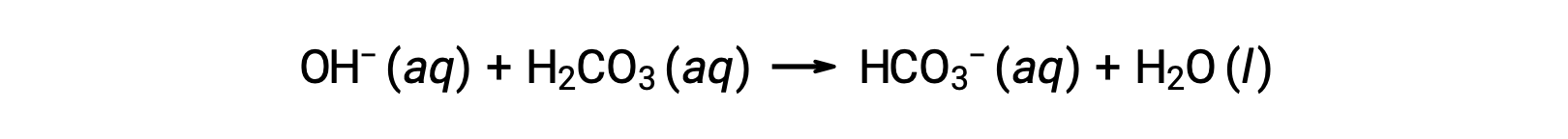
The added strong acid or base is thus effectively converted to the much weaker acid or base of the buffer pair (H3O+ is converted to H2CO3 and OH− is converted to HCO3−). The pH of human blood thus remains very near the value determined by the buffer pairs pKa, in this case, 7.35. Normal variations in blood pH are usually less than 0.1, and pH changes of 0.4 or greater are likely to be fatal.
This text is adapted from Openstax, Chemistry 2e, Section 14.6: Buffers.