Transcript
An Arrhenius acid is a substance that produces hydrogen ions when it dissolves in water, while a base produces hydroxide ions. Hydrogen ions immediately react with water to form hydronium ions, but for simplicity, we'll keep thinking of them as hydrogen ions. Depending on the amount of hydrogen ions or hydroxide ions in the solution, it is considered acidic or basic.
We measure the amount of acidity or basicity using pH, which is calculated as the negative log of the concentration of hydrogen ions. So, pH values under 7 are acidic, and pH values above 7 are basic. pH 7 is neutral.
Acids and bases are also compared based on their strength, which is different from their pH. An acid's strength is related to how easily the hydrogen ion dissociates from the anion, called the conjugate base. The same idea follows for a base in reference to the hydroxide ion and its conjugate acid. We can assign a value to that strength using the acid dissociation constant, or Ka.
Ka is defined using the concentrations of the non-dissociated acid and the dissociated hydrogen ions and conjugate base. You may often see this relationship represented as pKa, which is simply the negative log of the Ka. The smaller the pKa, the stronger the acid.
Some acids, like hydrochloric acid, are monoprotic, meaning that they can dissociate only one hydrogen ion. Polyprotic acids, like phosphoric acid, can dissociate several hydrogen ions. Each dissociation has its own pKa.
So, how can we determine pKa? One way is to perform an acid-base titration. Titration is performed by slowly adding a solution of known concentration to a solution of unknown concentration while observing the reaction between them. In this case, the acid reacts with the base in a neutralization reaction to form a salt and water.
So, if we want to measure the concentration of hydrogen ions in an acid, we can simply titrate with a strong base with a known hydroxide ion concentration until the acid is neutralized. To perform a titration accurately, the base needs to be standardized — meaning that you know the exact hydroxide ion concentration. This is not always straightforward.
For example, NaOH, which you will use in your experiment, is very hygroscopic, meaning that it absorbs water from the atmosphere. This happens to NaOH both as a solid and in solution. So, the true concentration of a NaOH solution may be lower than you would expect.
To determine the exact concentration of NaOH, we must first perform an acid-base titration. To do this, you must use the base to titrate an acid with a known concentration. Potassium hydrogen phthalate, KHP, is a non-hygroscopic acid, so we can accurately calculate its concentration from its mass.
We can see when the titration is complete — meaning that the acid is neutralized — by using a pH indicator like phenolphthalein. Phenolphthalein is neutral and colorless between about pH 0 and pH 8.
As the pH increases, two hydrogen ions dissociate. This anionic form is pink. So, when we begin the titration, the KHP solution is acidic and the phenolphthalein is colorless. As we add NaOH and the hydrogen ions are neutralized, the pH increases.
In this reaction, the solution is neutral when equal amounts of acid and base have been mixed together. After that, the addition of a little more NaOH makes the pH basic and the solution turns pink. This is referred to as the endpoint. If we know the moles of KHP and the volume of NaOH used to neutralize it, we can calculate the exact concentration of the base.
Once we have a standardized base, we can determine the pKa of an acid by titrating a known concentration of the acid with our standardized base while monitoring the pH. The plot of pH versus the volume of base added is called a titration curve. The curve usually follows an S or sigmoidal shape, where the inflection point of the steepest part of the curve denotes an equivalence point.
Here, the moles of hydroxide ions and dissociated hydrogen ions are equal. Like pKa, we'll see one equivalence point for each dissociated hydrogen ion. So, a monoprotic acid has only one equivalence point, and a triprotic acid has three.
When we perform the titration, we will know that we have passed the equivalence point when the pH indicator just barely turns from colorless to pink. This is called the titration endpoint. Like when we standardize the base, this is when the solution has a small excess of hydroxide ions and thus is slightly basic.
Another inflection point in the graph occurs halfway to the equivalence point. Here, the concentrations of the dissociated and non-dissociated acids are equal. Thus, the pH at this point is equal to the pKa. So, if we perform a titration and determine the equivalence point volume, then we can calculate pKa as the pH at half of this volume.
In this lab, you'll first standardize your base and then perform a titration using that standardized base to determine two pKa’s of a polyprotic acid.
Abstract
Acids and Bases
An Arrhenius acid produces hydrogen ions when it is dissolved in water:
HA + H2O → H+(aq) + A-(aq)
Here, HA is the non-dissociated acid, H+ is the hydrogen cation, and A- is the solvated anion — called the conjugate base. An Arrhenius base produces hydroxide ions when dissolved in water:
BOH + H2O → B+(aq) + OH-(aq)
Here, BOH is the non-dissociated base, OH- is the hydroxide ion, and B+ is the solvated cation — called the conjugate acid. A conjugate base is formed when an acid loses a hydrogen ion and has the potential to gain a hydrogen. The same follows for a conjugate acid, which is formed when a base loses a hydroxyl group and has the potential to regain it. Every acid has a conjugate base, and every base has a conjugate acid.
pH
pH is the degree of acidity of the solution and is a measure of the amount of hydrogen ions in a solution. The pH scale is logarithmic and runs from 0 to 14; aqueous solutions with a pH below 7 are described as acidic, and aqueous solutions with a pH above 7 are described as alkaline or basic. Solutions at pH 7 are considered neutral.
The pH of a solution is equal to the negative log base ten of the concentration of hydrogen ions in solution.

Water interacts strongly with the hydrogen ion because its strong positive charge attracts the negative pole of surrounding water molecules. In fact, they interact so strongly that they form a covalent bond and the H3O+ cation, called hydronium. The above equation is rewritten to reflect this.

For simplicity, we’ll refer to the concentration of hydrogen ions instead of hydronium ions when discussing pH. The lower the pH value of a solution, the more hydrogen ions that are present, and by extension, the more acidic the solution. For example, the pH of 1 mM of sulfuric acid is 2.75, whereas the pH of 1 mM of hydrochloric is 3.01. The concentration of hydrogen ions in the sulfuric acid solution is calculated as 1 × 10-2.75, whereas the concentration of hydrogen ions in the hydrochloric acid solution is 1 × 10-3.01. Thus, there are more hydrogen ions present in sulfuric acid, and it is more acidic. Remember, even though the pH of two solutions may vary by as little as half a pH value, due to the logarithmic nature of the pH scale, the amount of hydrogen varies greatly.
Strength of Acids and Bases
An acid’s strength is affected by the electronegativity of the conjugate base and the polarity of the acidic hydrogen. Strength, therefore, refers to how readily the hydrogen cation (H+) disassociates from the anion. Strong acids and bases dissociate entirely in aqueous solutions, whereas weak acids and bases only dissociate partially into their conjugate ions.
The dissociation constant, Ka, represents acid strength. Ka is calculated using the concentrations of the non-dissociated acid HA, and the concentrations of the hydrogen cations and the conjugate base, A-. Higher Ka values represent stronger acids, whereas smaller Ka values represent weaker acids.

Ka is numerically very small, and it is often reported in the form of pKa, which is the negative log base ten of Ka. Lower pKa values correspond to a stronger acid, whereas higher pKa values correspond to a weaker acid.

Some acids dissociate only one hydrogen ion and therefore have one pKa value. These acids are called monoprotic. However, some acids can dissociate more than one hydrogen ion and are called polyprotic. These acids have a pKa value for each hydrogen ion dissociation.
pKa can also be used to calculate the equilibrium pH of an acid-base reaction, as shown in the Henderson-Hasselbalch equation.
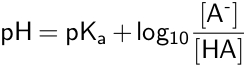
The Henderson-Hasselbalch equation is used to calculate pH, when the concentrations of the conjugate base and the weak acid are known, or to calculate the pKa if the pH and concentrations are known.
Titration
Acid-base reactions are quantitatively studied using titration. In a titration experiment, a solution of a known concentration, called a standard solution, is used to determine the concentration of another solution. For acid-base titrations, a standardized solution of base is slowly added to an acid of unknown concentration (or the acid is added to the base). The acid-base reaction is a neutralization reaction, which forms a salt and water. When the moles of hydrogen ions in the acid are equal to the moles of hydroxyl ions added from the base, the solution reaches neutral pH.
To perform an acid-base titration, the standardized base is slowly added to a stirring flask of the unknown acid using a burette, which enables the measurement of volume and the dropwise addition of base. The pH of the solution is closely monitored throughout the titration using a pH indicator added to the acid. Typically, phenolphthalein is used as the solution remains colorless until it becomes basic, turning a light pink.
As the titration approaches the equivalence point, which is when the moles of hydrogen ions equal the moles of hydroxyl ions added, the pH indicator temporarily changes color due to an excess of hydroxyl ions. When the flask is swirled, the pH indicator’s acidic color returns. The titration is complete and has reached its endpoint when a tiny excess of hydroxyl ions changes the indicator permanently to its basic color.
The titration curve is a plot of the pH of a solution versus the volume of standardized base added. The equivalence point is located at the inflection point of the curve, and it is calculated as the second derivative of the titration curve.
If an acid is polyprotic, it will have multiple equivalence points, one for each hydrogen ion dissociation. The pH at the halfway point to the equivalence point for monoprotic acids, or between equivalence points in the case of polyprotic acids, is equal to the pKa of the acid.
References
- Kotz, J.C., Treichel Jr, P.M., Townsend, J.R. (2012). Chemistry and Chemical Reactivity. Belmont, CA: Brooks/Cole, Cengage Learning.
- Silberberg, M.S. (2009). Chemistry: The Molecular Nature of Matter and Change. Boston, MA: McGraw-Hill.
- Harris, D.C. (2015). Quantitative Chemical Analysis. New York, NY: W.H. Freeman and Company.