Procedure
Dalton was only partially correct about the particles that make up matter. All matter is composed of atoms, and atoms are composed of three smaller subatomic particles: protons, neutrons, and electrons. These three particles account for the mass and the charge of an atom.
The Discovery of the Electron
The first clue about the subatomic structure came at the end of the 19th century when J.J. Thomson discovered the electron using a cathode ray tube. This apparatus consisted of a sealed glass tube, from which almost all the air had been removed, and that contained two metal electrodes. When high voltage was applied across the electrodes, a visible beam called a cathode ray appeared between them. This beam was deflected toward the positive charge and away from the negative charge, and was produced in the same way with identical properties when different metals were used for the electrodes. In similar experiments, the ray was simultaneously deflected by an applied magnetic field. Measurements of the extent of deflection and the magnetic field strength allowed Thomson to calculate the charge-to-mass ratio of the cathode ray particles. The results of these measurements indicated that these particles were much lighter than atoms. Based on his observations, Thomson proposed the following:
- The particles are attracted by positive (+) charges and repelled by negative (−) charges, so they must be negatively charged (like charges repel and unlike charges attract);
- The particles are less massive than atoms and indistinguishable, regardless of the source material, so they must be fundamental, subatomic constituents of all atoms.
- The volume occupied by an atom must consist of a large amount of empty space.
- A small, relatively heavy, positively charged body, the nucleus, must be at the center of each atom.
Thomson’s cathode ray particle is an electron, a negatively charged, subatomic particle with a mass more than 1000× less than that of an atom. The term “electron” was coined in 1891 by Irish physicist George Stoney, from “electric ion.”
In 1909, Robert A. Millikan calculated the charge of an electron by his “oil drop” experiments. Millikan created microscopic oil droplets, which could be electrically charged by friction as they formed or by using X-rays. These droplets initially fell due to gravity, but their downward progress could be slowed or even reversed by an electric field lower in the apparatus. By adjusting the electric field strength and making careful measurements and appropriate calculations, Millikan was able to determine the charge on individual drops to be 1.6 × 10−19 C (coulomb). Millikan concluded that this value must, therefore, be the fundamental charge of a single electron. Since the charge of an electron was now known due to Millikan’s research — and the charge-to-mass ratio was already known due to Thomson’s research (1.759 × 1011 C/kg) — the mass of the electron was determined to be 9.107 × 10−31 kg.
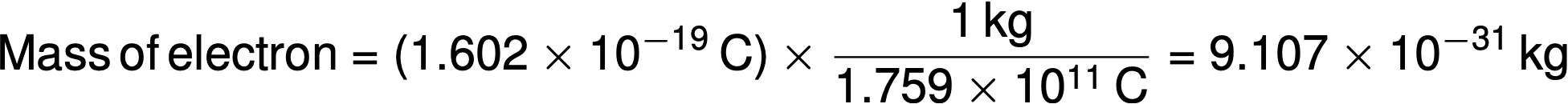
Rutherford’s Nuclear Model
Scientists had now established that the atom was not indivisible as Dalton had believed, and due to the work of Thomson, Millikan, and others, the charge and mass of the negative, subatomic particles — the electrons — were known. Scientists knew that the overall charge of an atom was neutral. However, the positively charged part of an atom was not yet well understood. In 1904, Thomson proposed the “plum pudding” model of atoms, which described a positively charged mass with an equal amount of negative charge in the form of electrons embedded in it, since all atoms are electrically neutral. A competing model had been proposed in 1903 by Hantaro Nagaoka, who postulated a Saturn-like atom, consisting of a positively charged sphere surrounded by a halo of electrons.
The next major development in understanding the atom came from Ernest Rutherford. He performed a series of experiments using a beam of high-speed, positively charged alpha particles (α particles) that were produced by the radioactive decay of radium. He aimed a beam of α particles at a very thin piece of gold foil and examined the resultant scattering of the α particles using a luminescent screen that glowed briefly when hit by an α particle. He observed that most particles passed right through the foil without being deflected at all. However, some were diverted slightly, and a very small number were deflected almost straight back toward the source.
From this, Rutherford then deduced the following: Because most of the fast-moving α particles passed through the gold atoms undeflected, they must have traveled through essentially empty space inside the atom. Alpha particles are positively charged, so deflections arose when they encountered another positive charge (like charges repel each other). Since like charges repel one another, the few positively charged α particles that changed paths abruptly must have hit, or closely approached, another body that also had a highly concentrated, positive charge. Since the deflections occurred a small fraction of the time, this charge only occupied a small amount of the space in the gold foil.
Analyzing a series of experiments, Rutherford drew two important conclusions:
This analysis led Rutherford to propose a model in which an atom consists of a very small, positively charged nucleus, in which most of the mass of the atom is concentrated, surrounded by the negatively charged electrons so that the atom is electrically neutral. After many more experiments, Rutherford also discovered that the nuclei of other elements contain the hydrogen nucleus as a “building block,” and he named this more fundamental particle the proton, the positively charged, subatomic particle found in the nucleus.
The Structure of an Atom
Protons are found in the nucleus of an atom and have a positive charge. The number of protons is equal to the atomic number on the periodic table and determines the identity of the element. Neutrons are also found in the nucleus. They have no charge, but they have the same mass as protons and thus contribute to the atomic mass of an atom. Electrons orbit around the nucleus in clouds. They have a negative charge and negligible mass, so they contribute to the overall charge of an atom, but not to its mass.
Neutrons
The nucleus was known to contain almost all of the mass of an atom, with the number of protons only providing half, or less, of that mass. Different proposals were made to explain what constituted the remaining mass, including the existence of neutral particles in the nucleus. It was not until 1932 that James Chadwick found evidence of neutrons, uncharged, subatomic particles with a mass approximately the same as that of protons.
The existence of the neutron also explained isotopes: They differ in mass because they have different numbers of neutrons, but they are chemically identical because they have the same number of protons.
Atomic Mass Unit (amu) and the fundamental unit of charge (e)
The nucleus contains the majority of an atom’s mass because protons and neutrons are much heavier than electrons, whereas electrons occupy almost all of an atom’s volume. The diameter of an atom is on the order of 10−10 m, whereas the diameter of the nucleus is roughly 10−15 m — about 100,000 times smaller. Atoms — and the protons, neutrons, and electrons that compose them — are extremely small. For example, a carbon atom weighs less than 2 × 10−23 g, and an electron has a charge of less than 2 × 10−19 C. When describing the properties of tiny objects such as atoms, appropriately small units of measure, such as the atomic mass unit (amu) and the fundamental unit of charge (e) are used. The amu is defined with regard to the most abundant isotope of carbon, atoms of which are assigned masses of exactly 12 amu. Thus, one amu is exactly 1/12 of the mass of one carbon-12 atom: 1 amu = 1.6605 × 10−24 g. The Dalton (Da) and the unified atomic mass unit (u) are alternative units that are equivalent to the amu.
The fundamental unit of charge (also called the elementary charge) equals the magnitude of the charge of an electron with e = 1.602 × 10−19 C. A proton has a mass of 1.0073 amu and a charge of 1+. A neutron is a slightly heavier particle with a mass 1.0087 amu and a charge of zero; as its name suggests, it is neutral. The electron has a charge of 1− and is a much lighter particle with a mass of about 0.00055 amu. For reference, it would take about 1800 electrons to equal the mass of one proton. The properties of these fundamental particles are summarized in the following table.
| Subatomic particle | Charge (C) | Unit charge | Mass (g) | Mass (amu) |
| Electron | −1.602 × 10−19 | 1− | 0.00091 × 10−24 | 0.00055 |
| Proton | 1.602 × 10−19 | 1+ | 1.67262 × 10−24 | 1.00727 |
| Neutron | 0 | 0 | 1.67493 × 10−24 | 1.00866 |
This text is adapted from Openstax, Chemistry 2e, Section 2.2: Evolution of Atomic Theory and Section 2.3: Atomic Structure and Symbolism.