Overview
The rate of a reaction is affected by the concentrations of reactants. Rate laws (differential rate laws) or rate equations are mathematical expressions describing the relationship between the rate of a chemical reaction and the concentration of its reactants.
For example, in a generic reaction aA + bB ⟶ products, where a and b are stoichiometric coefficients, the rate law can be written as:
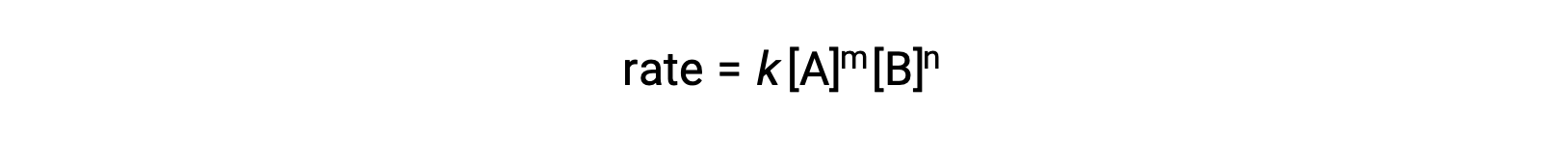
[A] and [B] represent the molar concentrations of reactants, and k is the rate constant, which is specific for a particular reaction at a specific temperature.
The exponents m and n are the reaction orders and are typically positive integers, though they can be fractions, negative, or zero.
The rate constant k and the reaction orders m and n are determined experimentally by observing how the rate of a reaction changes as the concentrations of the reactants are changed. The rate constant k is independent of the reactant concentrations, but varies with temperature.
The reaction orders in a rate law describe the mathematical dependence of the rate on reactant concentrations. Referring to the generic rate law (rate = k[A]m[B]n), the reaction is m order with respect to A and n order with respect to B. For example, if m = 1 and n = 2, the reaction is first order in A and second order in B. The overall reaction order is simply the sum of orders for each reactant. For the example rate law here, the reaction is third order overall (1 + 2 = 3).
A common experimental approach to the determination of rate laws is the method of initial rates. This method involves measuring reaction rates for multiple experimental trials carried out using different initial reactant concentrations. Comparing the measured rates for these trials permits determination of the reaction orders and, subsequently, the rate constant, which together are used to formulate a rate law.
Rate laws may exhibit fractional orders for some reactants, and negative reaction orders are sometimes observed when an increase in the concentration of one reactant causes a decrease in reaction rate. It is important to note that rate laws are determined by experiment only and are not reliably predicted by reaction stoichiometry.
The reaction order determines the relationship between the reaction rate and the concentration of reactants or products.
• In a zero-order reaction, the concentration of the reactants does not have any effect on the rate of the reaction, which remains constant throughout.
• In a first-order reaction, the reaction rate is directly and linearly proportional to the change in reactant concentration. As the reactant concentration decreases, the reaction rate also decreases proportionally.
• In second-order or higher-order reactions, the reaction rate is proportional to the exponential value of the reactants. Therefore, as the reaction progresses and the concentration of the reactants decreases, the reaction rate decreases exponentially.
This text is adapted from Openstax, Chemistry 2e, Section 12.3: Rate Laws.
Procedure
The rate of a reaction often depends on the reactant concentrations. For any reaction, the relationship between the reaction rate and the reactant concentrations can be expressed mathematically using a rate law or the rate equation.
In a rate law, k is the proportionality constant, or the rate constant, and n is the reaction order with respect to a single reactant, whose value is often an integer. In rate laws for multi-reactant reactions, the overall reaction order is the sum of all reactant orders.
For each reactant, the reaction rate, rate constant, concentration, and the reaction order are all determined experimentally. The rate law expresses the relationship between all these parameters.
Individual reactant orders commonly take the values 0, 1, or 2, and based on the overall reaction order, chemical reactions can be categorized as zero-order, first-order, or second-order reactions.
A single-reactant, or unimolecular, chemical reaction for which the reaction rate remains constant throughout its duration is a zero-order reaction. The reactant order in a zero-order reaction is zero, and according to the rate law, the reactant concentration is raised to the zeroth power.
Since the value of any number raised to the zeroth power is one, the reaction rate of a zero-order reaction is equal to the rate constant and, hence, independent of the reactant concentration. Therefore, in a zero-order reaction, even as the reactant's concentration decreases, the reaction rate does not slow down.
A unimolecular chemical reaction where the reaction rate is directly proportional to the reactant's concentration is a first-order reaction. The reactant order for a first-order reaction is one, and as per the rate law the reactant’s concentration is raised to the first power.
Since the value of any number raised to the power of one remains the same, the reaction rate of a first-order reaction directly depends on the reactant concentration. As the reactant concentration decreases, the reaction rate decreases proportionally in a linear manner.
A unimolecular chemical reaction where the reaction rate is dependent on the square of the reactant’s concentration is a second-order reaction. The reactant order is two, and the reactant concentration is raised to the second power.
Accordingly, the reaction rate in a second-order reaction directly depends on the square of the reactant concentration. As the reactant concentration decreases, the reaction rate decreases exponentially in a quadratic manner.