Overview
Chemical Kinetics and the Reaction Rate Law
Chemical kinetics refers to the rate or speed of a chemical reaction. The rate depends on the mechanism, complexity, and number of reactants in the reaction. Reactant concentration also plays a significant role in the rate of a reaction.
The rate law quantifies this relationship through experimentation. Each reactant contributes to the speed of the reaction by a factor known as the reaction order. This factor can range from zero to two, and it depends on the relationship of that reactant with the rate of the reaction.
r = k[A]m[B]n for aA + bB + ... → cC
In this equation, r is the reaction rate, k is the reaction rate constant, [A] and [B] are the concentrations of the reactants A and B, and m and n are the reaction orders of reactants A and B, respectively. The reaction rate, r, is defined as the change in concentration of the product with time and has units of moles per liter per second.
Order of a Reaction
The order of the reaction describes the power dependence that the concentration of reactants has on the reaction rate. The overall reaction order is the sum of the reaction orders for each of the reactants. It is important to remember that the reaction order is NOT related to the stoichiometric factors of the balanced chemical equation. In other words, stoichiometry cannot be used to determine the reaction orders for a chemical reaction. Reaction orders must be determined experimentally
To determine the reaction order, the concentration of the second reactant is held constant, while the concentration of the first reactant is varied. The time it takes for the reaction to occur is measured in seconds; however, this time does not correspond to the time it takes the reaction to go to completion. Instead, this is the time it takes for the reaction to start. The reaction times for the different reactant concentrations are then compared to determine the order. The same series of reactions are performed to determine the order of the second reactant, where the concentration of the first reactant is held constant and the concentration of the second reactant is varied.
By comparing the reaction times, the reaction order can be determined. For example, if the reaction time remains constant despite changes to reactant concentration, then the reactant is zero-order. This means that the rate of the reaction is equal to the reaction rate coefficient, k, which must have units of M/s. If the reaction time changes linearly with changes in concentration (i.e., doubling the concentration affects the reaction time by a factor of 2), then the reactant is first order. This means that the rate of the reaction is equal to the product of the reaction rate coefficient and the concentration of the reactant. In this case, k has units of 1/s. Finally, if the reaction time is affected by a factor of 4 when the concentration of a reactant is doubled, the reactant is second order. This means that the rate of the reaction is equal to the rate constant times the square of the reactant concentration, forming a quadratic relationship. In this case, the units of k must be 1/M⋅s.
The overall order of the reaction is the sum of the individual reaction orders. For example, if the reaction is first order with respect to A, then m = 1. And if the reaction is zero-order with respect to B, then n = 0. The overall order of the reaction is first order because m + n = 1.
Reaction Rate Constant
The rate constant, k, is specific to the reaction and temperature the reaction is performed. The rate constant is determined from the series of experiments. Using the measured rate and the initial reactant concentrations, the rate equation is used to solve for the rate constant. The rate constant is dependent on temperature and is defined by the Arrhenius equation. This equation describes the relationship between k and the activation energy, Ea, the temperature, T, and the ideal gas constant, R. The constant A is a constant of proportionality and is not to be confused with reactant A.
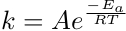
References
- Kotz, J.C., Treichel Jr, P.M., Townsend, J.R. (2012). Chemistry and Chemical Reactivity. Belmont, CA: Brooks/Cole, Cengage Learning.
- Silderberg, M.S. (2009). Chemistry: The Molecular Nature of Matter and Change. Boston, MA: McGraw Hill.
Procedure
The measure of how fast a reaction proceeds is called the reaction rate. For a single-step reaction, the rate is equal to the change in concentration of each reactant or product over time multiplied by the inverse of the corresponding stoichiometric coefficient. You can think of the change in concentration over time as the concentration at time t minus the starting concentration divided by t. Reaction rates are always positive, so the reactant expressions have negative signs. These rates may not be equal in multi-step reactions, but we can still use this relationship as an estimate for the overall reaction.
The rate law, or rate equation, describes the relationship between the speed of the reaction and the reactant concentrations. In this equation, k is the rate constant, A and B are the two reactants, and m and n are their respective reaction orders. Reaction order describes the relationship between the concentration of a reactant and the rate and is not related to stoichiometry. It is vital to remember that the reaction order is not the same as the coefficient of the reactant in the balanced equation.
When a reaction involves two or more reactants, we must consider the reaction order for each reactant. The overall reaction order is equal to the sum of the reactants' reaction orders. For example, if reactant A is first-order and reactant B is zero-order, the overall reaction order is one. The most common reaction orders in simple reactions are zero-order, first-order, and second-order. Let's go through them using a unimolecular reaction as an example.
If the reaction is zero order, the reactant concentration has no effect on the reaction rate. Thus, the reaction rate is equal to k, the rate constant. A graph of the reaction rate with respect to concentration is a horizontal line.
In first order relationships, the reactant concentration is linearly related to the reaction rate. Thus, the rate is equal to the rate constant times the reactant concentration. A graph of the reaction rate with respect to concentration will be linear, with k as the slope.
If the reaction is second order, there is a quadratic relationship between the concentration and the rate. Thus, the rate is equal to the rate constant times the concentration squared. A graph of the rate with respect to concentration will be parabolic, with k as the slope.
The rate constant, k, is a temperature-dependent value that relates the activation energy of the reaction to the reaction rate. You will explore this in the next lab experiment.
Reaction rates are typically given in moles per liter per second. The rate has the same units, regardless of the reaction order. Thus, the rate constant has different units, depending on the reaction order. For example, if A is first order and B is zero order, then we have one instance of moles per liter in the rate equation. Therefore, the rate constant must be in inverse seconds.
So how do we determine reaction order? The reaction order must be determined experimentally using a series of tests. If you have two reactants, one method is to hold the concentration of one reactant constant, vary the other, and time how long it takes to make a certain amount of product. The same process is repeated for the second reactant. You can then estimate the order for each reactant by plotting rate versus the reactants' varying concentration, and seeing whether it looks like a zero, first, or second-order graph. Matching your data to the corresponding rate equation will also let you calculate k.
In this lab, you will determine the reaction orders of two reactants by varying their concentrations and timing how long it takes the reaction to progress to turning the solution opaque.