Overview
Although gaseous molecules travel at tremendous speeds (hundreds of meters per second), they collide with other gaseous molecules and travel in many different directions before reaching the desired target. At room temperature, a gaseous molecule will experience billions of collisions per second. The mean free path is the average distance a molecule travels between collisions. The mean free path increases with decreasing pressure; in general, the mean free path for a gaseous molecule will be hundreds of times the diameter of the molecule
In general, when a sample of gas is introduced to one part of a closed container, its molecules very quickly disperse throughout the container; this process by which molecules disperse in space in response to differences in concentration is called diffusion. The gaseous atoms or molecules are, of course, unaware of any concentration gradient; they simply move randomly — regions of higher concentration have more particles than regions of lower concentrations, and so a net movement of species from high to low concentration areas takes place. In a closed environment, diffusion will ultimately result in equal concentrations of gas throughout. The gaseous atoms and molecules continue to move, but since their concentrations are the same in both bulbs, the rates of transfer between the bulbs are equal (no net transfer of molecules occurs). The amount of gas passing through some area per unit time is the rate of diffusion.
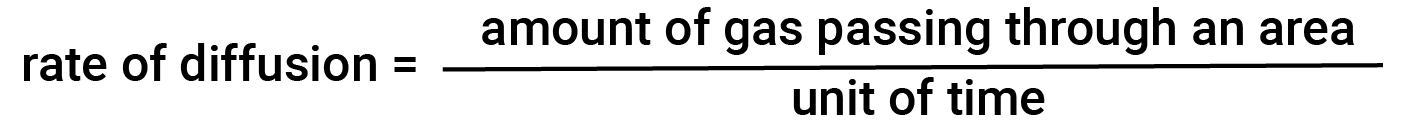
The diffusion rate depends on several factors: the concentration gradient (the increase or decrease in concentration from one point to another), the amount of surface area available for diffusion, and the distance the gas particles must travel.
A process involving the movement of gaseous species similar to diffusion is effusion, the escape of gas molecules through a tiny hole, such as a pinhole in a balloon into a vacuum. Although diffusion and effusion rates both depend on the molar mass of the gas involved, their rates are not equal; however, the ratios of their rates are the same.
If a mixture of gases is placed in a container with porous walls, the gases effuse through the small openings in the walls. The lighter gases pass through the small openings more rapidly (at a higher rate) than the heavier one. In 1832, Thomas Graham studied the rates of effusion of different gases and formulated Graham’s law of effusion: The rate of effusion of a gas is inversely proportional to the square root of the mass of its particles:

This means that if two gases, A and B, are at the same temperature and pressure, the ratio of their effusion rates is inversely proportional to the ratio of the square roots of the masses of their particles:

The relationship indicates that the lighter gas has a higher effusion rate.
For example, a helium-filled rubber balloon deflates faster than an air-filled one because the rate of effusion through the pores of the rubber is faster for the lighter helium atoms than for the air molecules.
This text is adapted from Openstax, Chemistry 2e, Section 9.4: Effusion and Diffusion of Gases.
Procedure
A closed bottle of perfume contains a high concentration of gaseous aromatic molecules that are constantly moving and randomly colliding. Meanwhile, the air outside of the bottle contains essentially none of these molecules.
On opening the bottle, a concentration gradient is established between these high- and low-concentration regions. The molecules continue moving randomly, with overall movement from the high-concentration region to the low-concentration region.
The spontaneous mixing and spreading of liquids or gases in response to a concentration gradient is called molecular diffusion.
Diffusion is a slow process. Even though gas particles travel at high speeds, the numerous collisions cause frequent changes in speed and direction.
The average distance a particle travels between collisions is known as its mean free path. For a gas particle, its mean free path is influenced by the particle density, which also affects the pressure.
As the particle density increases, so does the collision frequency. Thus, their mean free path is shorter. Likewise, as the particle density decreases, so does the collision frequency, leading to a longer mean free path.
Different gases diffuse at different rates, depending on the speed of the gas particles. Since the root-mean-square, or RMS, speed and the molar mass of a gas are inversely related, lighter gases diffuse faster than heavier gases.
Consider a glass tube between reservoirs of equal amounts of ammonia and hydrogen chloride gas.
When the diffusing gases meet, they react to form a ring of ammonium chloride. The ring is closer to the hydrogen chloride end of the tube because the lighter ammonia molecules traveled farther down the tube than the heavier hydrogen chloride molecules in the same amount of time.
Effusion is another process that involves the movement of gas molecules. It is the ability of gas molecules to travel through a hole whose diameter is much smaller than the mean free path of the gas itself in response to a pressure difference.
This is why helium balloons eventually deflate — the helium gradually effuses through tiny pores in the balloon material.
Like diffusion, the rate of effusion is dependent on the RMS speed and molar mass of the gas. Specifically, the rate of effusion is inversely proportional to the square root of the molar mass of the gas. Therefore, heavier gases effuse more slowly than lighter ones.
For any two gases, the ratio of their effusion rates is the square root of the inverse ratio of their molar masses. This is called Graham’s law of effusion.
Consider two balloons inflated to the same pressure — one filled with helium and the other with oxygen. Helium has a lower molar mass than oxygen, as shown by the helium balloon’s buoyancy in air.
Applying Graham’s law to helium and oxygen suggests that helium effuses 2.8 times faster than oxygen. Thus, the helium balloon deflates faster than the oxygen balloon.