Overview
Unless individual gases chemically react with each other, the individual gases in a mixture of gases do not affect each other’s pressure. Each gas in a mixture exerts the same pressure that it would exert if it were present alone in the container. The pressure exerted by each individual gas in a mixture is called its partial pressure.
This means that in a mixture containing three different gases A, B, and C, if PA is the partial pressure of gas A; PB is the partial pressure of gas B; PC is the partial pressure of gas C; then the total pressure is given by equation 1:
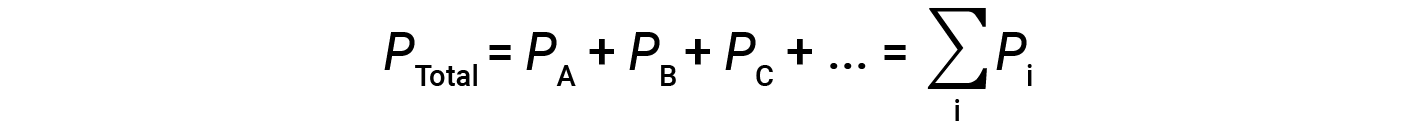
This is Dalton’s law of partial pressures: The total pressure of a mixture of ideal gases is equal to the sum of the partial pressures of the component gases.
Let nA, nB, and nC be the number of moles of each of the gases in the mixture. If each gas obeys the ideal-gas equation, the partial pressure can be written as:
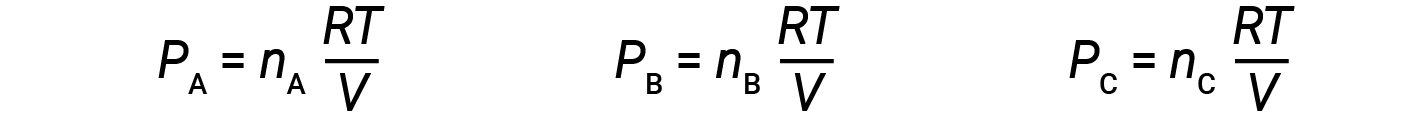
Since all gases are at the same temperature and occupy the same volume, substituting into equation 1 gives:
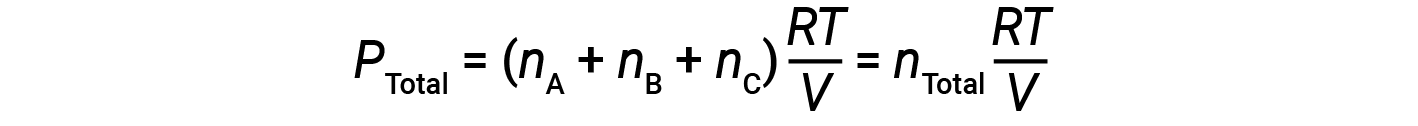
The equation indicates that at constant temperature and constant volume, the total pressure of a gas sample is determined by the total number of moles of gas present.
For mixtures of gases, it is convenient to introduce a quantity called the mole fraction, χ, which is defined as the number of moles of a particular substance in a mixture divided by the total number of moles of all substances present. Mathematically, the mole fraction of a substance A in a mixture with B and C is expressed as
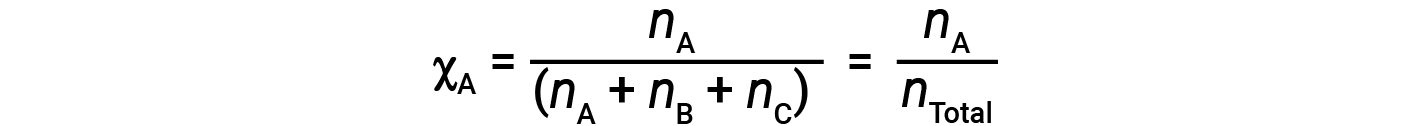
Similarly, the mole fraction of B and C are;
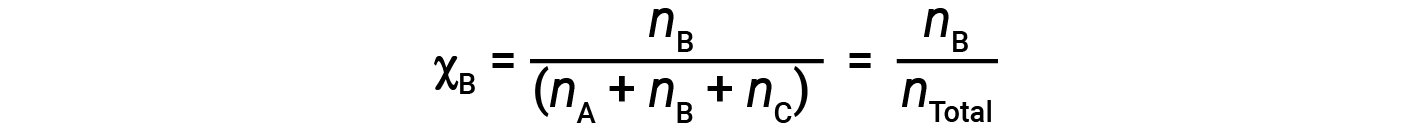
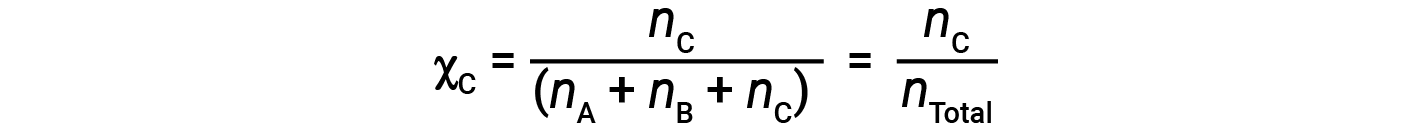
Combining the equation for the mole fraction of A and the equation for partial pressure gives:
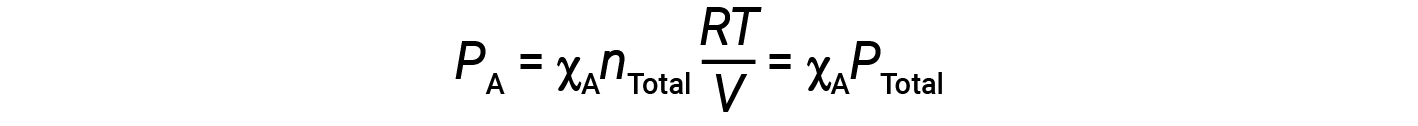
The partial pressure of gas A is related to the total pressure of the gas mixture via its mole fraction.
In other words, the pressure of a gas in a mixture of gases is the product of its mole fraction and the total pressure of the mixture.
This text is adapted from Openstax, Chemistry 2e, Section 9.3: Stoichiometry of Gaseous Substances, Mixtures, and Reactions.
Procedure
The pressure of a pure gas is the sum of molecular collisions between its particles and surrounding surfaces. A gas sample with fewer particles in a given volume exerts a lower pressure than a sample with more particles in the same volume.
But what is the pressure of a mixture of different gases? For a multicomponent gas mixture, the pressure is the sum of collisions from all gas molecules.
It is assumed that each component in the mixture exerts its own pressure that is independent of the other gases present. The pressure from any individual component is called its partial pressure.
The total pressure of the ideal gas mixture equals the sum of the partial pressures of its components. This observation is Dalton's law of partial pressures.
By applying the ideal gas law, the partial pressures of the individual gas components are substituted with measurable variables.
Since the gases in the mixture occupy the same volume and are at the same temperature, the equation can be simplified.
The sum of the moles of the individual components equals the total number of moles of all gas components, ntotal. Therefore, the total pressure of the gas mixture is equal to ntotal multiplied by the constant RT over V.
The number of moles of a component divided by the total moles in the mixture is the mole fraction.
Rearranging the mole fraction for total moles, and substituting ntotal in Dalton’s law of partial pressures yields expressions for the total pressure. Rearranging again, the partial pressure of a gas in a mixture is the product of its mole fraction and the total pressure of the mixture.
So, in a gas mixture, the partial pressure of any component, i, is equal to the mole fraction of i multiplied by the total pressure.
As an example calculation, suppose a container filled with two gases, helium, and argon, is 40% by volume argon. This implies that the mole fraction of argon is 0.4. If the total pressure is 4 atm, what is the partial pressure of helium?
Using the equation for the partial pressure of a gas, the partial pressure of argon is equal to its mole fraction multiplied by the total pressure. Thus, 0.4 times 4 atm gives the partial pressure of argon as 1.6 atm.
Since the sum of partial pressures equals the total pressure, the equation can be rearranged so that the partial pressure of argon can be subtracted from the total pressure. Thus, the partial pressure of helium is 2.4 atm.