Procedure
The relative amounts of reactants and products represented in a balanced chemical equation are often referred to as stoichiometric amounts. However, in reality, the reactants are not always present in the stoichiometric amounts indicated by the balanced equation.
In a chemical reaction, the reactant which gets consumed first, and limits the amount of product formed, is the limiting reactant, while the other substance becomes the excess reactant. An excess of one or more reactants is often used to ensure the complete conversion of the other reactant into the product.
Consider the reaction for the formation of water represented by the equation:
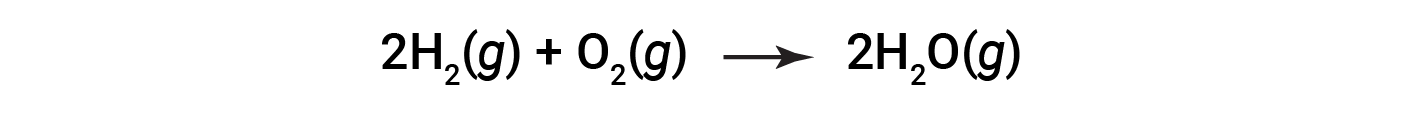
The balanced equation shows the hydrogen and oxygen react in a 2:1 stoichiometric ratio. If these reactants are provided in any other amounts, one of the reactants will nearly always be entirely consumed, thus limiting the amount of product that may be generated. This substance is the limiting reactant, and the other substance is the excess reactant. Identifying the limiting and excess reactants for a given situation requires computing the molar amounts of each reactant provided and comparing them to the stoichiometric amounts represented in the balanced chemical equation.
Stoichiometry indicates that two moles of hydrogen and one mole of oxygen react to produce two moles of water; that is, hydrogen and oxygen combine in a 2:1 ratio. Imagine if 5 moles of hydrogen and 2 moles of oxygen are present. The ratio of the reactants is now 5:2 (or 2.5:1), which is greater than the stoichiometric ratio of 2:1. Hydrogen, therefore, is present in excess, and oxygen is the limiting reactant. Reaction of all the provided oxygen (2 mol) will consume 4 mol of the 5 mol of hydrogen provided, leaving 1 mol of hydrogen unreacted. Computing the molar amounts of each reactant provided and comparing them to the stoichiometric amounts represented in the balanced chemical equation is one way of identifying the limiting and excess reactant.
An alternative approach involves calculating the amount of product formed in moles from each reactant, as per the reaction’s stoichiometry, and then comparing the amounts. The reactant that produces a lesser amount of product moles is the limiting reactant. For example, the complete reaction of five moles of hydrogen would yield:

Similarly, the complete reaction of two moles of oxygen would yield:

Oxygen produces fewer moles of water, and therefore, oxygen is the limiting reactant. Oxygen will be completely consumed once 4 moles of H2O have been produced. The stoichiometry between hydrogen and oxygen being 2:1, four moles of hydrogen are needed to react with two moles of oxygen.

Thus, (5 mol H2 − 4 mol H2 = 1 mol H2), one mole of unreacted hydrogen will remain once this reaction is complete.
This text is adapted from OpenStax, Chemistry 2e, Section 4.4: Reaction Yield.